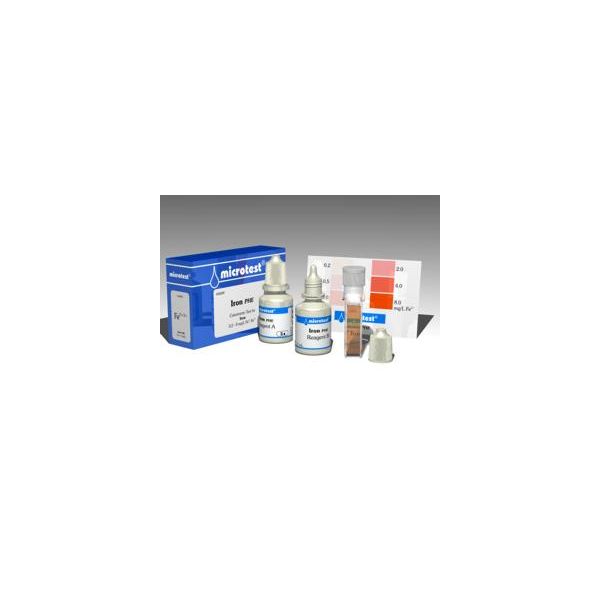
microtest Iron PHE
$88.66
Colorimetric Test for Iron
0.2 – 8 mg/L Fe2+/Fe3+
Availability: Back Order
SKU
AQ656000
| Contents | Reagent A, 15 mL Reagent B, 15 mL Cuvette, 4 mL Colour chart Instruction |
| Analytical Method | 1,10-Phenanthroline: In the pH range 2.9 – 3.5 ferrous iron (Fe2+) forms an orange-red complex with 1,10-phenanthroline. The intensity of its colour is related to the iron concentration. Ferric iron (Fe3+) is reduced to Fe2+ prior to the measurement. |
| Number of Tests | 80 |
| Interferences | The test is suitable for freshwater and seawater. Interferences are caused by cyanide, nitrite, chromium, zinc (>10 mg/L), cobalt, copper (>5 mg/L) and nickel (>2 mg/L). Only dissolved iron will be determined. |
| Measuring Range | The standard measuring range is 0.2 – 8 mg/L. The colour comparator has 6 colour fields: 0.2–0.5–1–2–4–8 mg/L. For higher iron concentrations, the sample has to be diluted. A 1:4 sample dilution can be carried out easily, thus extending the measuring range to 32 mg/L. |
| Shelf Life | 2 years |
| Applications | Determination of iron in ground waters (bore water); monitoring of steel corrosion in industrial water treatment. |
Write Your Own Review